REACH regulations : the SVHC product list

Safic-Alcan is a global specialty chemicals distributor that offers a direct access to a large and diverse portfolio of innovative products. Our regulatory support accompanies our partners not only to ensure they remain compliant in any market, but also in anticipating upcoming industry trends.
What does SVHC mean ?
SVHC stands for Substances of very high concern, they are :
- Carcinogens category 1A or 1B
- Mutagens category 1A or 1B
- Toxic to reproduction category 1A or 1B
- Persistent, bioaccumulative and toxic
- very persistent and very bioaccumulative
- Substances with an equivalent level of concern to those listed above (e.g. endocrine disruptors, respiratory sensitisers)
Who and how decides if a chemical is SVHCs ?
Member States, or ECHA at the request of the European Commission, may propose a substance to be identified as a substance of very high concern (SVHC) by preparing a dossier in accordance with the requirements set out in Annex XV to REACH.
The intention is published in the registry of intentions before the proposal is submitted, to inform interested parties in advance of the submission.
After publication of the proposal, interested parties can comment on it or provide further information during the 45-day consultation. Comments can be made on the properties of the substance, its uses and alternatives.
When comments are received that provide new information or challenge the basis for the identification as an SVHC, both the proposal and the comments are referred to the Member State Committee (MSC) to agree on the identification of the substance as an SVHC.
If the committee reaches a unanimous agreement, the substance is added to the Candidate List. If the committee does not reach a unanimous agreement, the matter is referred to the Commission.
![]()
SAFIC-ALCAN advises you comment during the public consultation, so that decisions are based on as much information as possible.
When is the SVHC list updated ?
The list is updated every six months, often in January and in July. It has 224 entries since the last update 10/06/2022. It can be consulted from the ECHA website.
Am I concerned ?
Companies may have legal obligations resulting from the inclusion of substances in the Candidate List. These obligations, which are effective from the date of inclusion, refer not only to the listed substances on their own or in mixtures but also to their presence in articles. Definition of substances, mixtures and articles is in Article 3, REACH¹.
![]()
SAFIC-ALCAN advises you to clearly identify whether your product is a substance, a mixture or an article under REACH.
What is the obligation ?
The obligations including informing customers and consumers, notifying ECHA, submitting information in SCIP Database, providing Safety Data Sheets, minimizing releases. Requirements can be different according to the article’s composition. The details can be found on ECHA’s dedicated page².
![]()
SAFIC ALCAN reminds you that as long as the SVHC substance is not in the authorization list (Annexe XIV of REACH), it is not prohibited.
What is the relation between SVHC and Authorisation ?
The authorisation process aims to ensure that SVHCs are progressively replaced by less dangerous substances or technologies where technically and economically feasible alternatives are available.
ECHA regularly assesses the substances from the Candidate List to determine which ones should be included in the Authorisation List as a priority. The prioritisation is based on information on the intrinsic properties, wide dispersive use or high volumes that fall within the scope of the authorisation requirement. ECHA launches a three months consultation as part of the process.
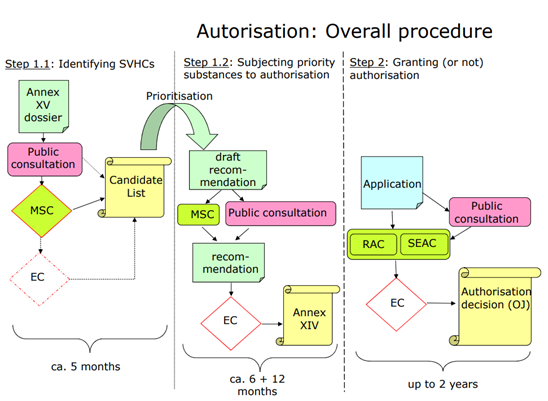
What is the impact if a substance is in the authorisation list ?
Companies that want to continue using a substance included in the Authorisation List after the sunset date need to prepare an application for authorisation and submit it before the latest application date.
Companies also need to pay for the authorisation application. Standard fees for applications for an authorisation are as follow :
- Base fee EUR 54 100
- Additional fee per substance EUR 10 820
- Additional fee per use EUR 48 690
![]()
SAFIC ALCAN advise you start to look for substitute solution as soon as the substance is included in the SVHC list. Because the main aim is to phase out these substances.
Sources :
Glossary :
CSR: Chemical safety report
ECHA: European Chemicals Agency
EEA: The European Economic Area
EU: European Union
MSC: Member State Committee
PBT: persistent, bioaccumulative and toxic
REACH: Registration, Evaluation and Authorisation of Chemicals
SCIP: Substances of Concern In articles as such or in complex objects (Products)
SVHC: Substance of Very High Concern
UVCB (unknown or variable composition, complex reaction products or of biological materials)
vPvB: very persistent and very bioaccumulative
WFD: Waste Framework Directive






